Air Ideal Gas
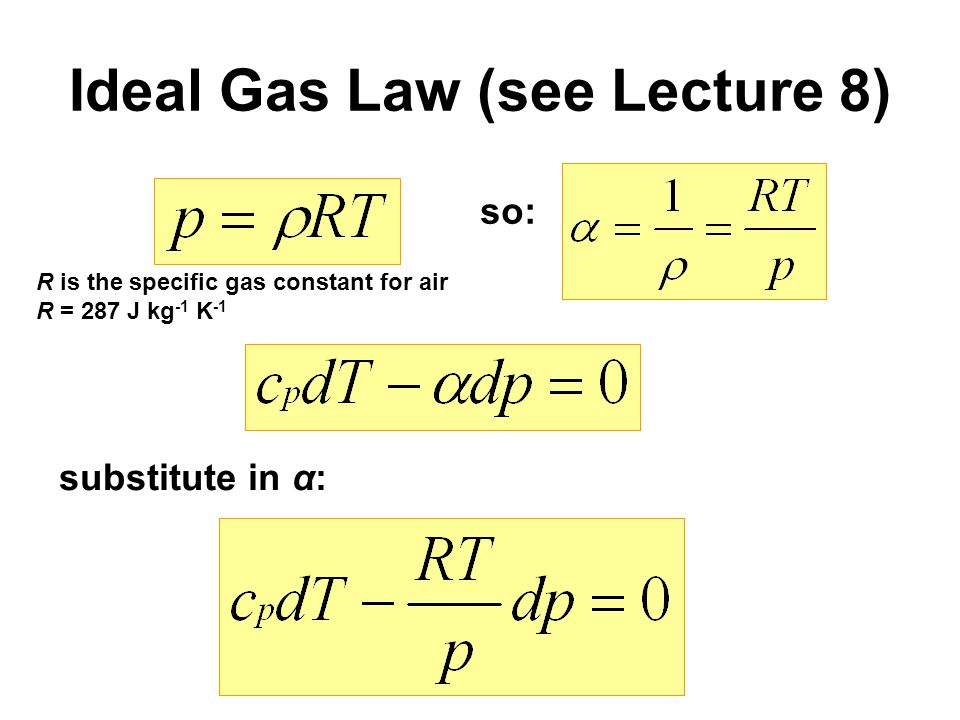
R is the ideal gas constant;.

Air ideal gas. Aug 17, 17 · It is no longer necessary to distinguish between ideal and real gas methods since real gas methods are easy to implement using today’s computational tools Volume Subject Area Oil and Gas Applications Topics Compressors, Equations of state, Fluids This content is only available via PDF. An ideal gas is a theoretical gas model, in which a gas is represented by many randomly moving point particles that interact with each other only perfectly elastically, that is when a collision between any two particles occurs, their kinetic energy remains the same and does not convert into any other form of energy such as potential energy or heat. Thermodynamics part 3 Kelvin scale and Ideal gas law example Thermodynamics part 4 Moles and the ideal gas law Thermodynamics part 5 Molar ideal gas law problem What is the ideal gas law?.
5 4 Entropy Changes in an Ideal Gas VW, S & B 65 66, 71 Many aerospace applications involve flow of gases (eg, air) and we thus examine the entropy relations for ideal gas behavior The starting point is form (a) of the combined first and second law,. These all come together as the ideal gas law pV = nRT (1) = m M RT = mRT where R is the universal gas constant 145 J K 1, pis pressure, Tis temperature, Mis molar weight of the gas, Vis volume, mis mass and n= m=Mis the molar abundance of a fixed collection of matter (an air parcel) The specific gas constant Ris related to the universal. The ideal gas law in terms of R is PmRTV , where P is the absolute pressure of the gas, V is the volume occupied by the gas, m is the mass of the gas, and T is the absolute temperature of the gas For air in SI units, air kJ 143 kmol K kJ J 2870 kg kg K kg K 27 kmol R Ru M For air in English units, air ft lbf lbmol R ft lbf.
Jun 11, 09 · Dry air contains roughly (by volume) * 7808% nitrogen * 95% oxygen * 093% argon * 0038% carbon dioxide All the above are relatively small, light, nonpolar molecules, characteristics. Feb 08, 03 · First, you need to work in absolute pressures, not gauge pressures for the ideal gas law 175 psig is about 190 psia (add 147 psi to your gauge pressure, technically the 147 applies to sea level but you are likely fine unless you are in Denver). Monitoring & controlling flue gas temperature, CO, CO2 & Percentage of oxyzen in flue gas CLCSS 610 LPG Fired Bead Making Furnace % Better temperature, improve product quality Preheating of secondary air CLCSS **Encon Thermal Engineering Pvt Ltd 611 Horizontal Flat and Bent Glass Electric Furnace for Tempering with Automatic Controller and.
Oct 02, 13 · The Ideal Gas Equation Before we look at the Ideal Gas Equation, let us state the four gas variables and one constant for a better understandingThe four gas variables are pressure (P), volume (V), number of mole of gas (n), and temperature (T) Lastly, the constant in the equation shown below is R, known as the the gas constant, which will be discussed in depth further later. Entropy of an Ideal Gas The entropy S of a monoatomic ideal gas can be expressed in a famous equation called the SackurTetrode equation where N = number of atoms k = Boltzmann's constant V = volume U = internal energy h = Planck's constant One of the things which can be determined directly from this equation is the change in entropy during an isothermal expansion. Reference State U = 0 and S o = 0 for an ideal gas at K The IG Property Calculator uses the Shomate Equation and constants obtained from the NIST Webbook in November, 14 Click on this box to close Join Learn Thermodynamics Advantage Use R134a.
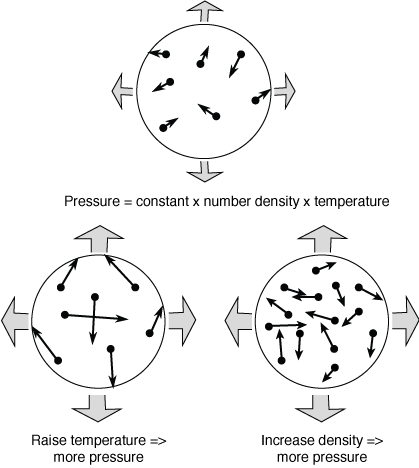
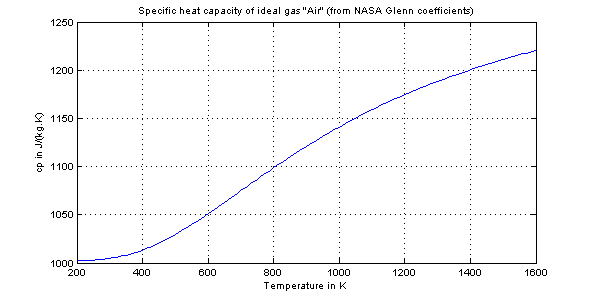
Air as an ideal gas flows through the compressor and heat exchanger shown in the diagram below A separate liquid water stream also flows through the heat exchanger The data given are for operation at steadystateStray heat transfer to the surroundings can be neglected, as can all kinetic and potential energy changes. Dec 28, · The ideal gas law relates the state variables pressure, temperature and volume for an ideal gas In an ideal gas, the gas molecules are treated as point particles interacting in perfectly elastic collisions, they are all relatively far apart and intermolecular forces can be ignored. Specific Heat Capacities of Air The nominal values used for air at 300 K are C P = 100 kJ/kgK, C v = 0718 kJ/kgK,, and k = 14 However they are all functions of temperature, and with the extremely high temperature range experienced in internal combustion and gas turbine engines one can obtain significant errors.
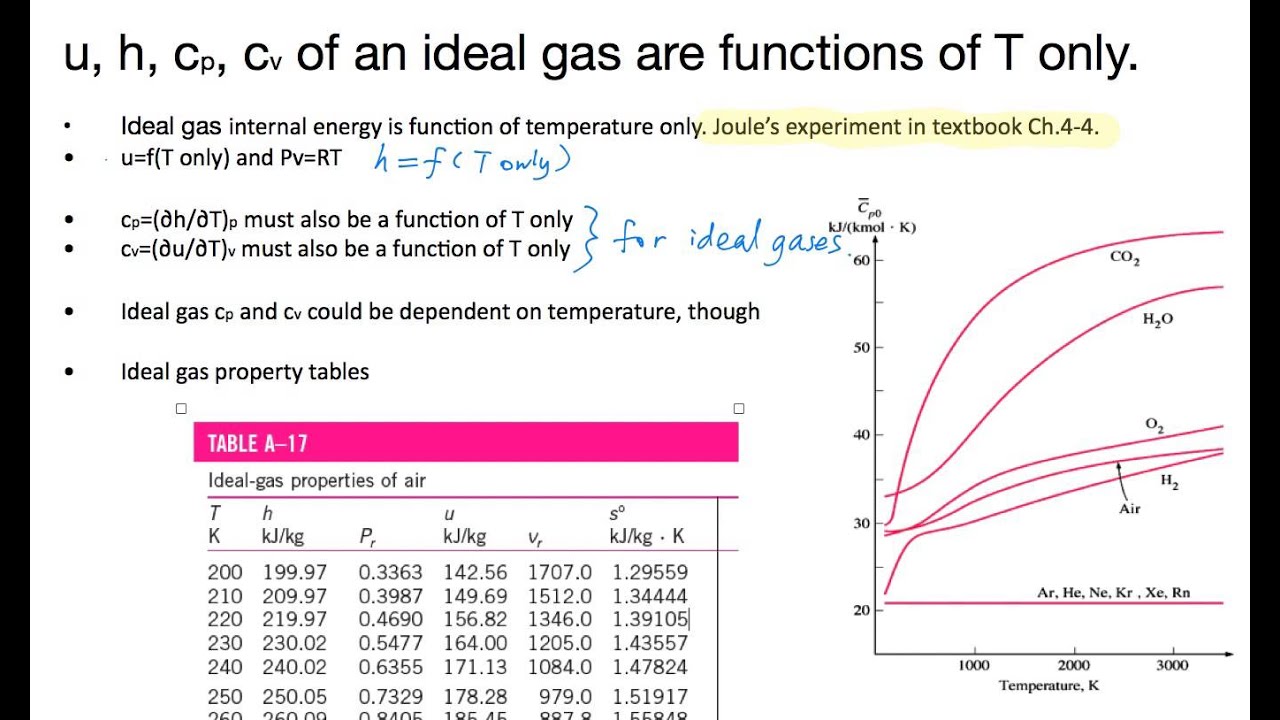
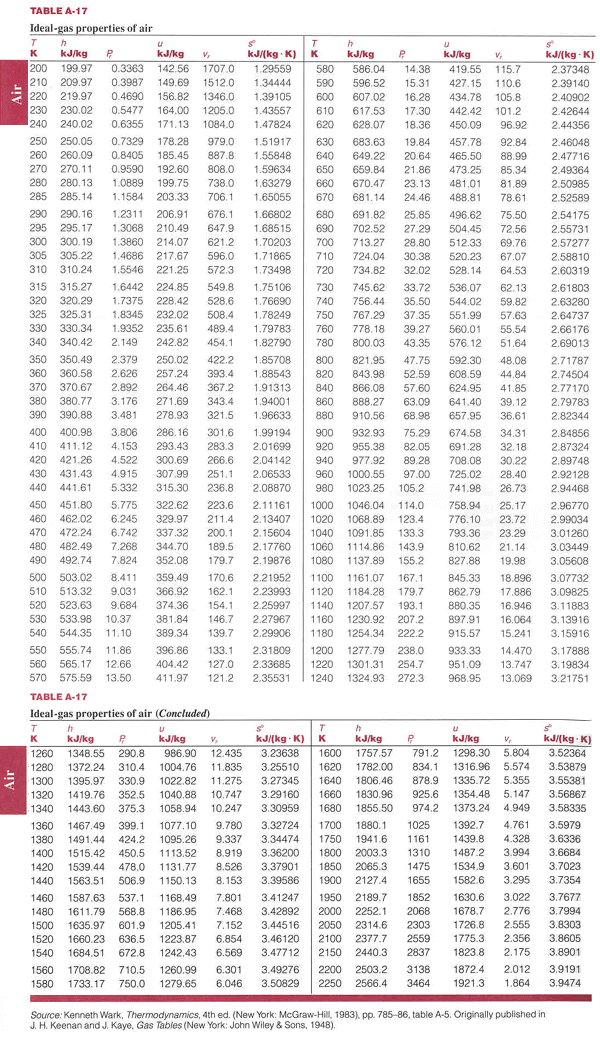
AIR IDEAL has an ergonomic design and is userfriendly, easy to operate and programmable, allowing sequential air sampling In an environment of increasingly strict regulation, the AIR IDEAL aerobiocollector meets the most stringent parameters CE, UL and CSA marked, the AIR IDEAL operates according to the impaction principle recommended by the. Appendix E Ideal Gas Properties of Air Ideal gas properties of air are provided in Table E1 The specific internal energy provided in Table E1 is computed by integration of the ideal gas specific heat capacity at constant volume ref T v T ucTdT and the specific enthalpy, h, provided in Table E1 is computed by integration of the ideal gas. Use data from Table 2.
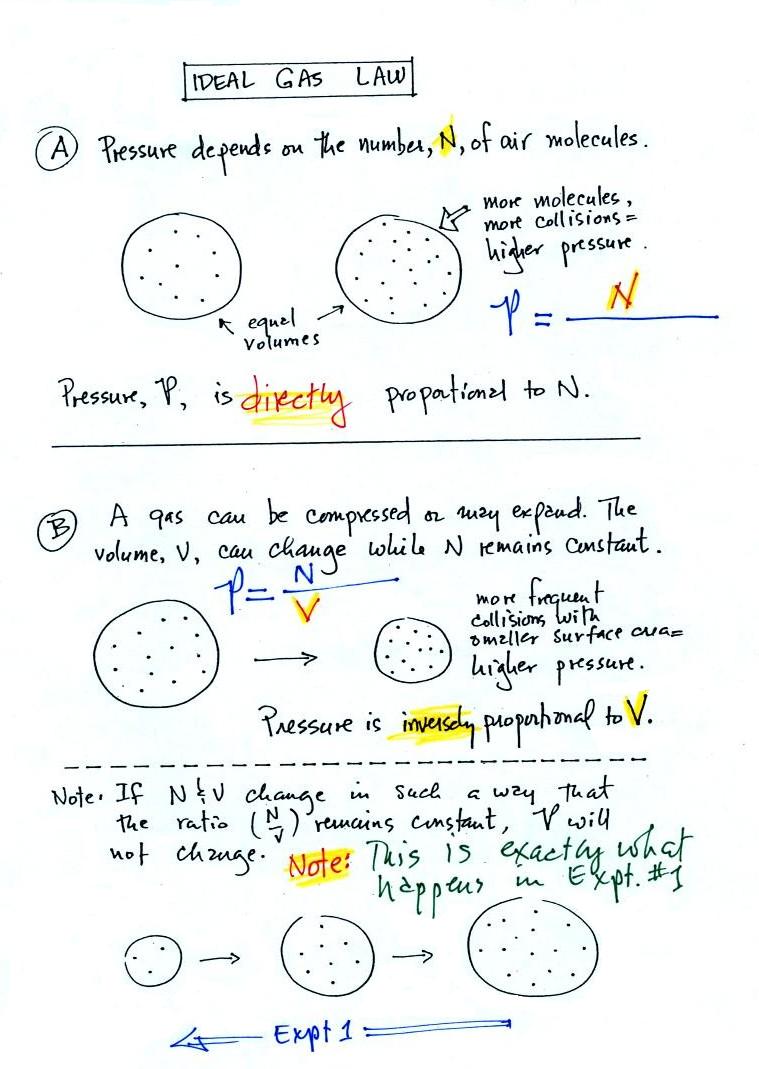
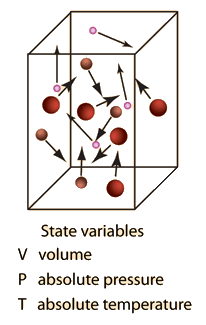
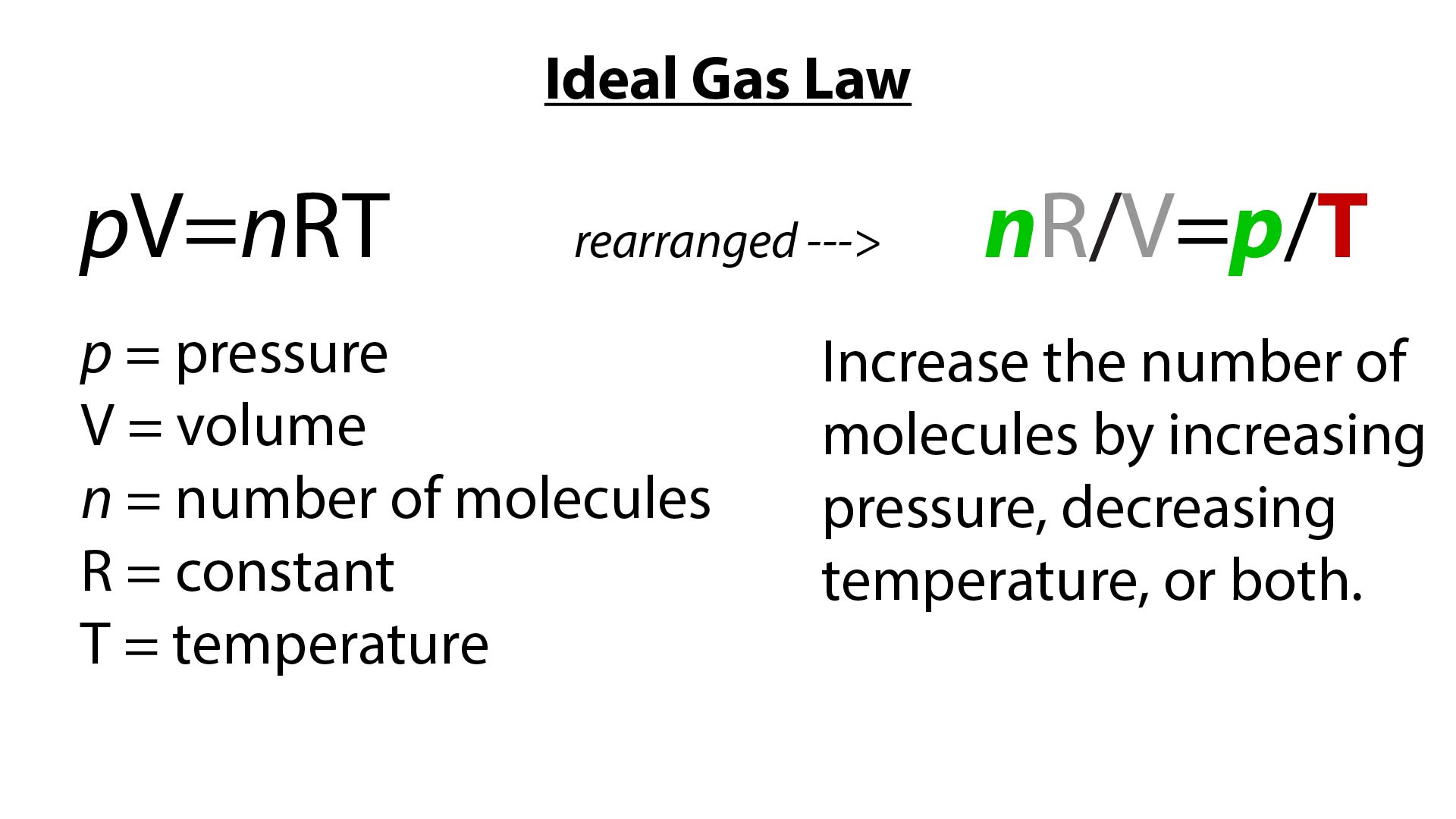
Where p is pressure (Pa = kg m –1 s –2), V is the volume (m 3), N is the number of moles, R* is the gas constant (14 J K –1 mole –1), and T is the temperature (K) Note also that both sides of the Ideal Gas Law equation have the dimension of energy (J = kg m 2 s –2) Recall that a mole is 602 x 10 23 molecules (Avagodro’s Number) Equation 21 is a form of the ideal gas law. The ideal gas law PV = Nk B T is called the ideal gas law It is the equation of state, which elates the quantities V, P, and T Most real gases at ordinary temperatures and pressures obey the ideal gas law The ideal gas law can be rewritten as PV = nN A k B T = nRT. T is the temperature of the gas, measured in Kelvins.
Air Ideal Gas Properties Calculator The Property of Ideal Air That You Know * Temperature (K) Enthalpy h (kJ/kg) Reduced Pressure Pr Internal Energy u (kJ/kg) Reduced Volume vr Standart Entropy s° (kJ/kgK). Morris Jenkins has provided air conditioning services and furnace repair to the Charlotte North Carolina area since 1958 Heating and air conditioning contractor providing air conditioner repair, new system installation, heat pump repair, gas pak service, maintenance, and more. Apr 03, 19 · Ideal Gases Versus Real Gases The Ideal Gas Law applies to ideal gasesAn ideal gas contains molecules of a negligible size that have an average molar kinetic energy that depends only on temperature Intermolecular forces and molecular size are not considered by the Ideal Gas Law The Ideal Gas Law applies best to monoatomic gases at low pressure and high temperature.
The Gas Constant (R) In PV = nRT The gas constant (R) is also known as the universal, molar, or ideal gas constant This gas constant referred to as a physical constant that is introduced in different fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation. Table SI Specific Heats for Ideal Gases in SI Units 4 Table A3SI Ideal Gas Properties of Air in SI Units 10 Table SI Ideal Gas Properties of N 2 in SI Units 15 Table A5SI Ideal Gas Properties of O 2 in SI Units Table A6SI Ideal Gas Properties of H 2 in SI Units 26 Table SI Ideal Gas Properties of CO 2 in SI Units 31 Table A8SI. Air gas constant R J/kg · K, k The speed of sound in water c = r E v ρ = s × Pa kg / m 3 = m / s The speed of sound in the air c = √ kRT = p () ( J / kg · K) (K) = m / s • The speed of.
Determine the speed of sound for the air (using the ideal gas approximation) and water at C water bulk modulus E v × Pa, density ρ kg/m ;. The ideal gas law states that PV = NkT, where P is the absolute pressure of a gas, V is the volume it occupies, N is the number of atoms and molecules in the gas, and T is its absolute temperature. Nov 07, 16 · Ideal gas law equation The properties of an ideal gas are all summarized in one formula of the form pV = nRT where p is the pressure of the gas, measured in Pa;.
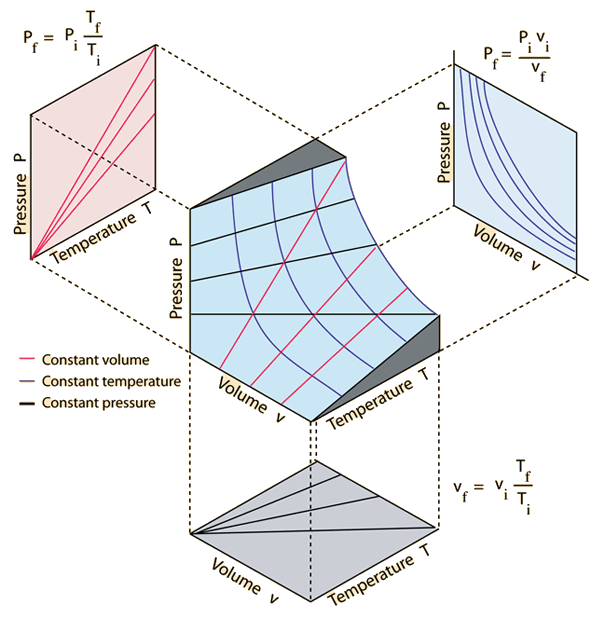
Jul 30, 02 · An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics The requirement of zero interaction can often be relaxed if, for example, the. The air density can be calculated with a transformation of the ideal gas law (5) to ρ = p / (R T) (7) ρ = ( (50 lb/in 2 147 lb/in 2 )*144 in 2 /ft 2 ) / (1716 ftlb/slug o R* (70 460) °R) = slugs/ft3 The weight of the air is the product of specific weight and the air volume. Pressure, temperature and volume for an ideal or perfect gas like air with water vapor or moist air In a perfect or ideal gas the correlations between pressure, volume, temperature and quantity of gas can be expressed by the Ideal Gas Law The Universal Gas Constant, Ru is independent of the particular gas and is the same for all "perfect" gases, and is included in of The Ideal Gas Law.
Apr 30, 21 · An ideal gas would have a value of 1 for that ratio at all temperatures and pressures, and the graph would simply be a horizontal line As can be seen, deviations from an ideal gas occur As the pressure begins to rise, the attractive forces cause the volume of the gas to be less than expected and the value of \(\frac{PV}{RT}\) drops under 1. May 10, 21 · "Gamma" is just a number whose value depends on the state of the gas For air, gamma = 14 for standard day conditions "Gamma" appears in several equations which relate pressure, temperature, and volume during a simple compression or expansion process Because the value of "gamma" just depends on the state of the gas, there are tables of these. This is the currently selected item The MaxwellBoltzmann distribution.
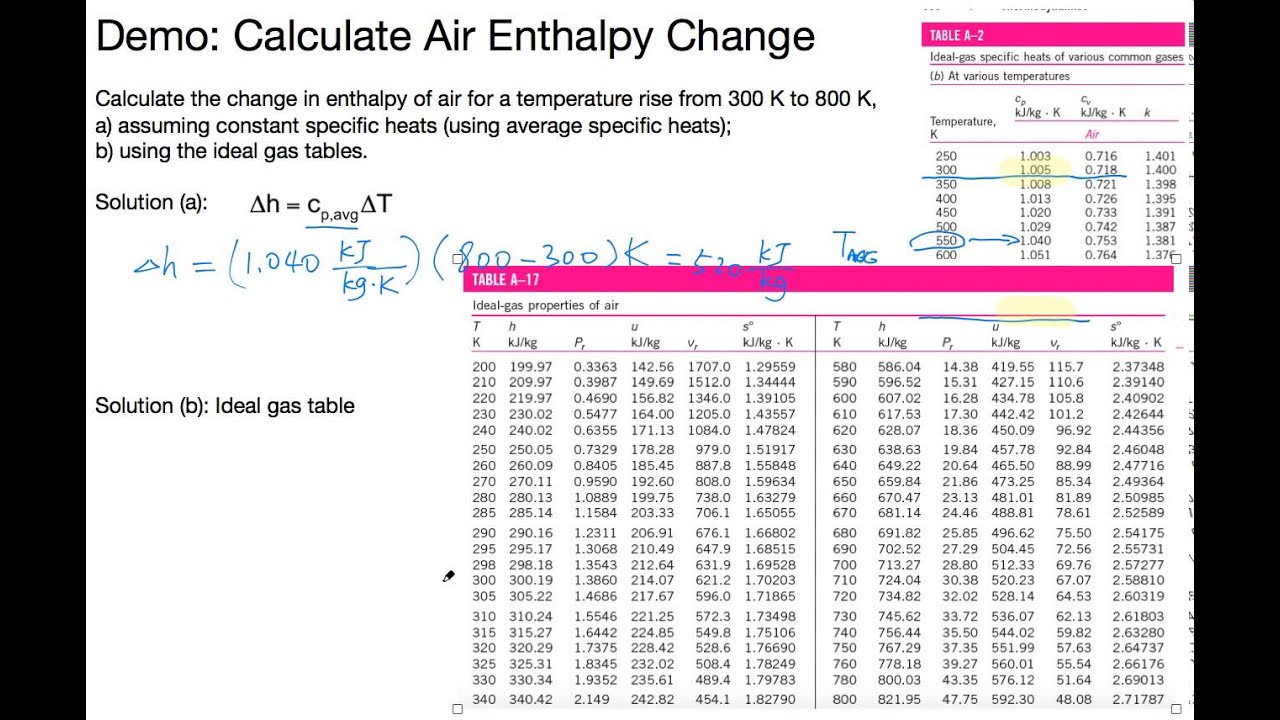
Question PROBLEM SET IDEAL GAS LAW 1 A sealed bottle contains air at 2 °C and a pressure of 1 x10 Pa The temperature is raised to 10 °C Calculate the new pressure 2 A gas has pressure x 10° Pa and volume 23 x103 m² The pressure is reduced to 45 x 106 Pa at constant temperature Calculate the new volume of the gas 3. Write the ideal gas law for the football in the locker room Solve for , the constants that won't change as the air cools Write the ideal gas law for the football on the field Substitute from before Recognize that the volume does not change, so those terms cancel Convert from absolute pressure to gauge pressure. Idealgas specific heats of various common gases (a) At 300 K Gas constant, Rc p c v Gas Formula kJ/kg·K kJ/kg·K kJ/kg·K k Air — 1005 0718 1400 Argon Ar 081 053 1667 Butane C 4H 10 1091 Carbon dioxide CO 2 018 0846 0657 12 Carbon monoxide CO 1040 0744 1400 Ethane C 2H 6 147 1186.
This question cannot be answered with a simple yes or no The error involved in treating water vapor as an ideal gas is calculated and plotted in this picture It is. Density = Mass / Volume The reason we know that the hot air is less dense than cool air is due to the Ideal Gas Law The Ideal Gas Law states that the Pressure times the Volume is equal to the number of molecules times the gas constant (R) times the Temperature Sometimes if you think of it in this equation it is a little easier to understand. , ideal (universal) gas constant, 1446 J/(mol·K), molar mass of dry air, kg/mol Temperature at altitude meters above sea level is approximated by the following formula (only valid inside the troposphere, no more than ~18 km above Earth's surface (and lower away from Equator)).
The Ideal gas law describes the behavior of a hypothetical real or ideal gas under various conditions Emile Clapeyron proposed it in 14 Statement Ideal gas law states that “for a given amount of gas, when the volume of the gas is compressed, the temperature of the gas increases. The ideal gas law is pV = nRT , where n is the number of moles, and R is universal gas constant The value of R depends on the units involved, but is usually stated with SI units as R = 14 J/mol·K. Ideal gases involve molecules that have no attraction between each other, and they don’t have a volume, both of which don’t occur in nature However, the Ideal Gas Law works well for gases at Earth’s atmosphere’s temperatures and pressures, at least those within 25 km of the Earth’s surface So for our needs, this equation is handy.
Consider air as an ideal gas There is a piston cylinder apparatus containing this air, and the air is compressed from State 1 to State 2 Model the air in the cylinder as a closed system, a control mass A Can the following process occur adiabatically?. Dec 06, 19 · The real gas that acts most like an ideal gas is helium This is because helium, unlike most gases, exists as a single atom, which makes the van der Waals dispersion forces as low as possible Another factor is that helium, like other noble gases, has a. V is the volume of the gas, measured in m³;.
An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly eleastic and in which there are no intermolecular attractive forces One can visualize it as a collection of perfectly hard spheres which collide but which otherwise do. Ideal gas theory is very important for analysis of processes because in most of the situations moisture content is extracted in the form of water vapor, which behaves as an ideal gas An ideal gas can be described in terms of three parameters the volume that it occupies, the pressure that it exerts, and its temperature. N is the amount of substance, measured in moles;.
May 23, 19 · The Ideal gas law is also known as general gas law As the name states the law is applicable under the ideal conditions, not to real gases The law correlates the pressure, volume, temperature, and amount of gas It was first formulated by French physicist Émile Clapeyron in. Ideal Gas Law This law combines the relationships between p, V, T and mass, and gives a number to the constant!. Ideal gas is a gas that obeys ideal gas law and specific heats of an ideal gas are function of temperature alone For example if you consider air as an ideal gas Cp=1005 f(T) and similarly Cv=0718g(T) So, internal energy of ideal gas can be simply calculated from du=integral{Cv dT} and enthalpy of an ideal gas is dh=integral{CpdT}.
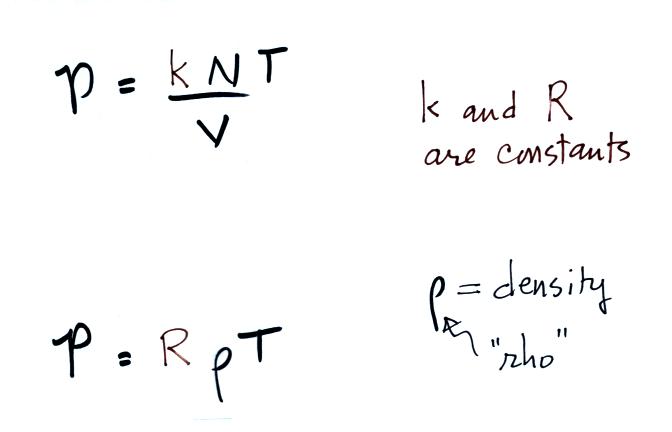
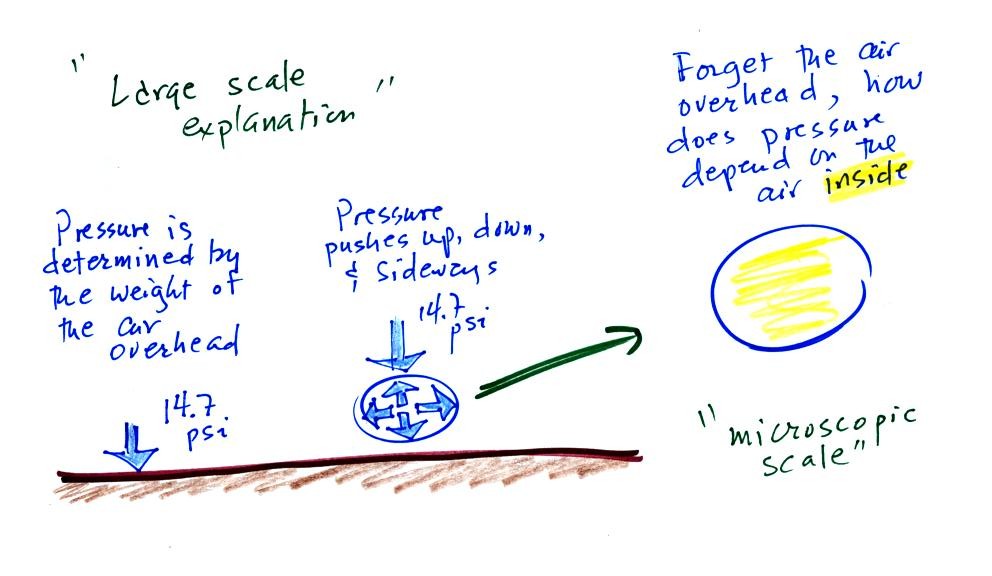
Lecture 6 Ideal Gas Law Rising And Sinking Air
Lecture 6 Ideal Gas Law Rising And Sinking Air

Internal Energy Of Ideal Gases Tec Science
Air Ideal Gas のギャラリー

Ideal Gas Law Problem Mass Of Air In A Room Youtube
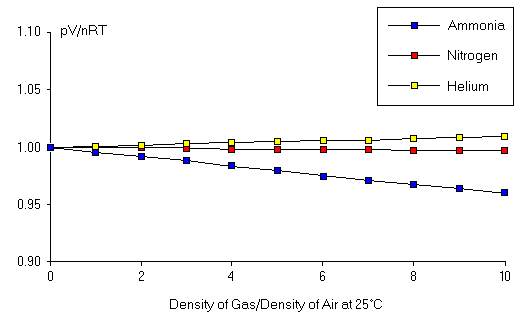
Validity Of The Ideal Gas Law

Ideal Gas Law Lab Moles Of Air In Classroom

Hw5 Sol Temperature Heat

Oneclass A Volume Of Air Assumed To Be An Ideal Gas Is First Cooled Without Changing Its Volume An

Ideal Gas Law Concept Development Studies In Chemistry 12 Openstax Cnx

Solved Air Ideal Gas At Pressure 100 Kpa And Temperatur Chegg Com

Thermodynamics The Ideal Gas Law Shmoop

Chemistry Ideal Gases Ppt Download
Lecture 6 Ideal Gas Law Rising And Sinking Air
Variation Of Dimensionless Parameter X X Ch4 Dpth X X 0 Ch4 0 With Download Scientific Diagram
Air Parcels And The Ideal Gas Law Science Pickle

How Does The Ideal Gas Law Relate To Leak Rates Ateq Corp Leak Testing United States
Planetary Science

Thermodynamics The Ideal Gas Law Shmoop
Ideal Gas Properties U H Cp Cv Youtube

Solved Solve This Problem Using Ees And The Ideal Gas Mod Chegg Com
Calculate Enthalpy Change Of An Ideal Gas System Youtube
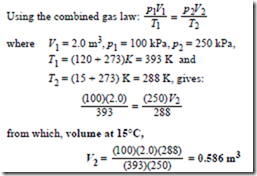
Ideal Gas Laws Worked Problems On The Characteristic Gas Equation And Further Worked Problems On The Characteristic Gas Equation Hvac Machinery

Ideal Gas Law Ck 12 Foundation

Ideal Gas Law Calculator

Pressure Distributions Of Air Ideal Gas Equation Of State For K W Sst Download Scientific Diagram

4 36 Kg Kmol Since Propane Is Heavier Than Air Molar Mass Ideal Gas

Ideal Gas Law Its Effects On Leak Testing Zaxis Inc
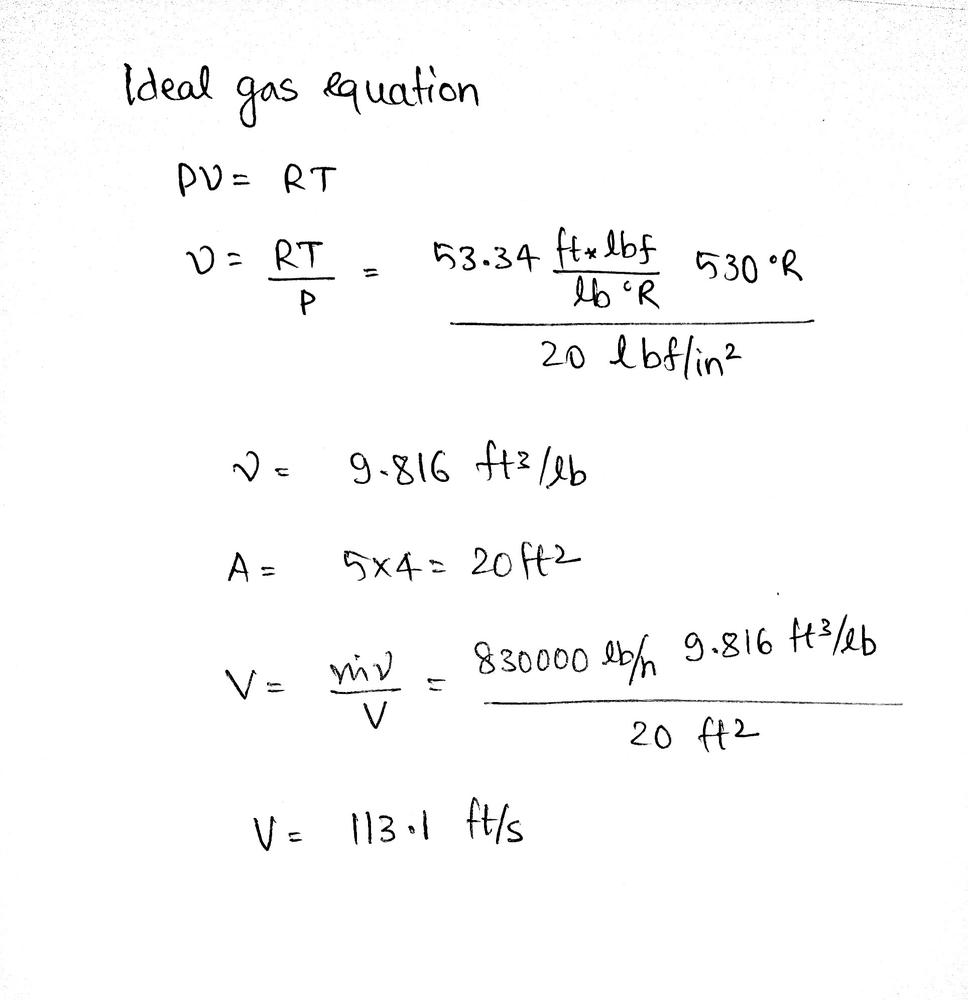
Air Modeled As An Ideal Gas Enters A Combustion Chamber At Math Lbf In 2 Math And Math 70 Circ Math F Through A Rectangular Duct 5 Ft By 4 Ft If The Mass Flow Rate Of The Air
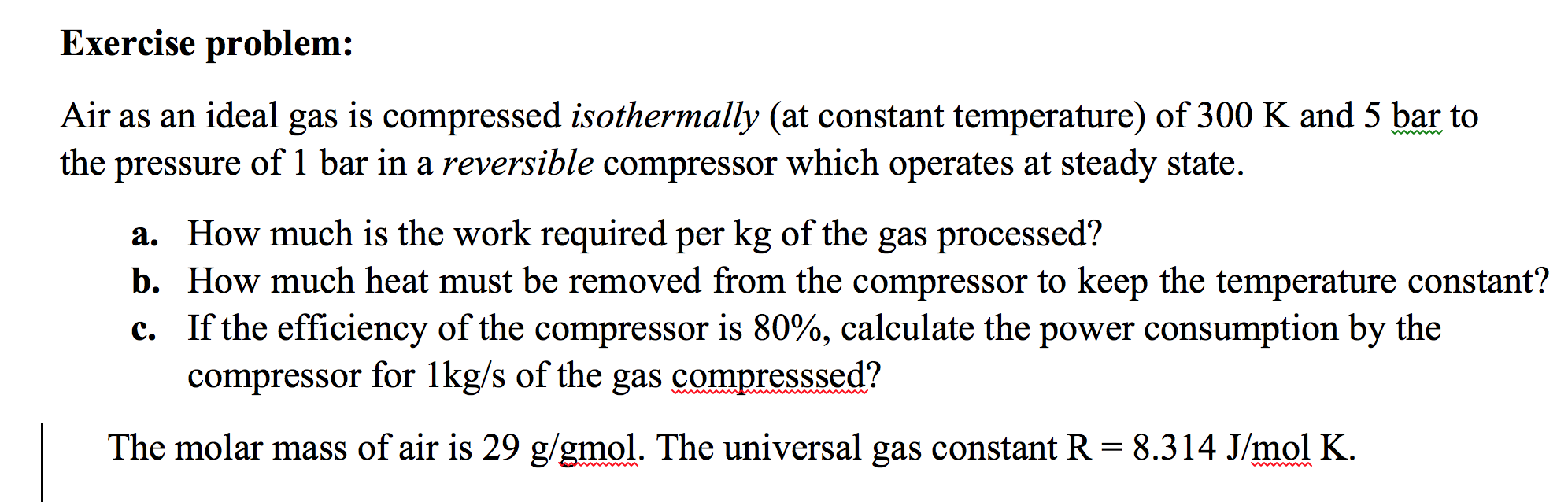
Solved Air As An Ideal Gas Is Compressed Isothermally At Chegg Com
Ideal Gas Law

Solved Iii How Well Does The Ideal Gas Law Desc

Area Of Usability Of The Ideal Gas Model For The Calculation Of Download Scientific Diagram
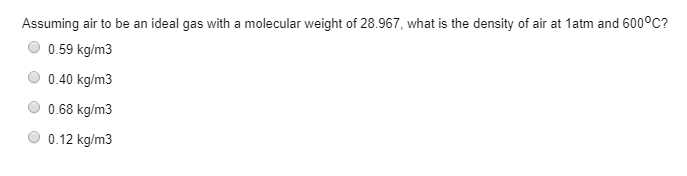
Solved Assuming Air To Be An Ideal Gas With A Molecular W Chegg Com
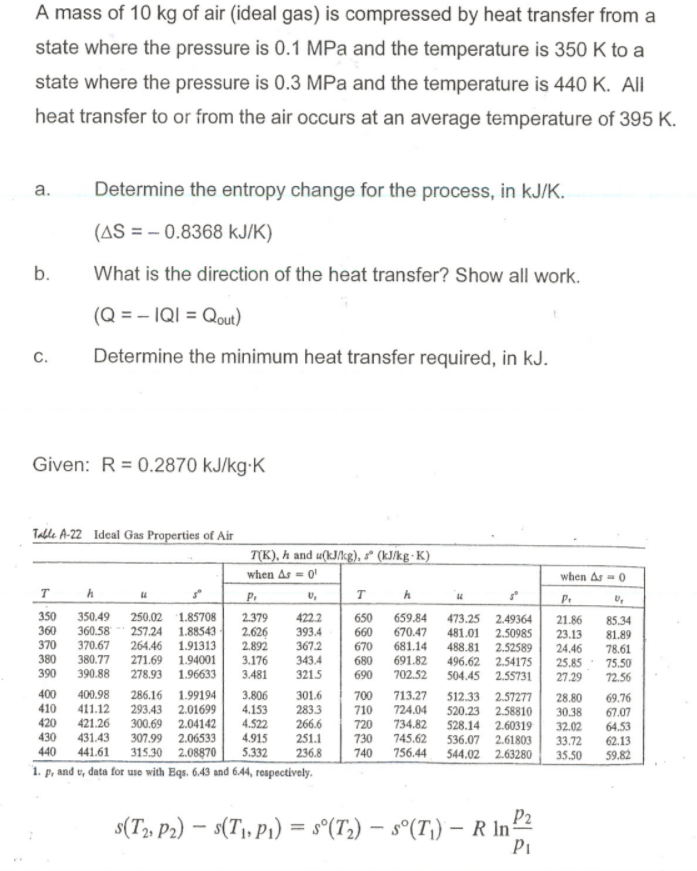
Solved A Mass Of 10 Kg Of Air Ideal Gas Is Compressed B Chegg Com

Thermodynamics Problems

Equation Of State

Fundamental Of Gases Ideal Gas Law The Behavior Of Chemicals In Air With Respect To Temperature And Pressure Can Be Assumed To Be Ideal In The Chemical Ppt Download
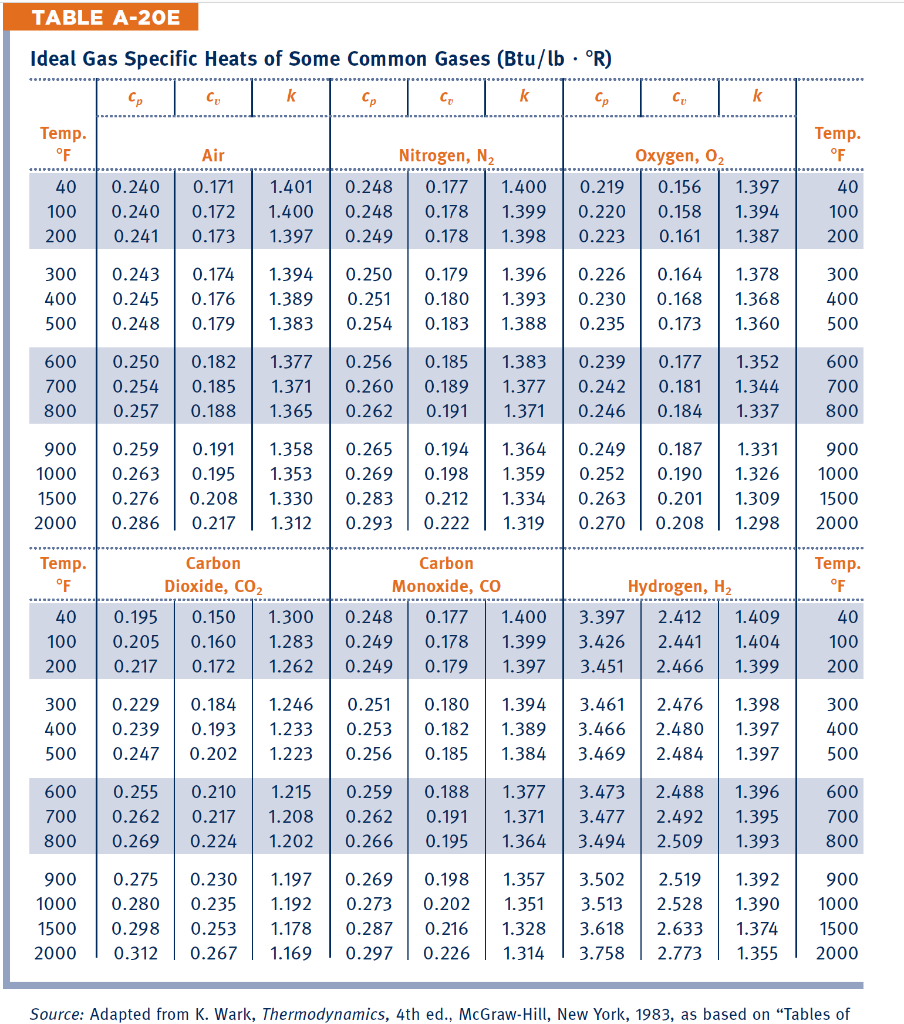
Sp 1 A Rigid Tank Contains 4 Lbm Of Air At 14 69 Chegg Com
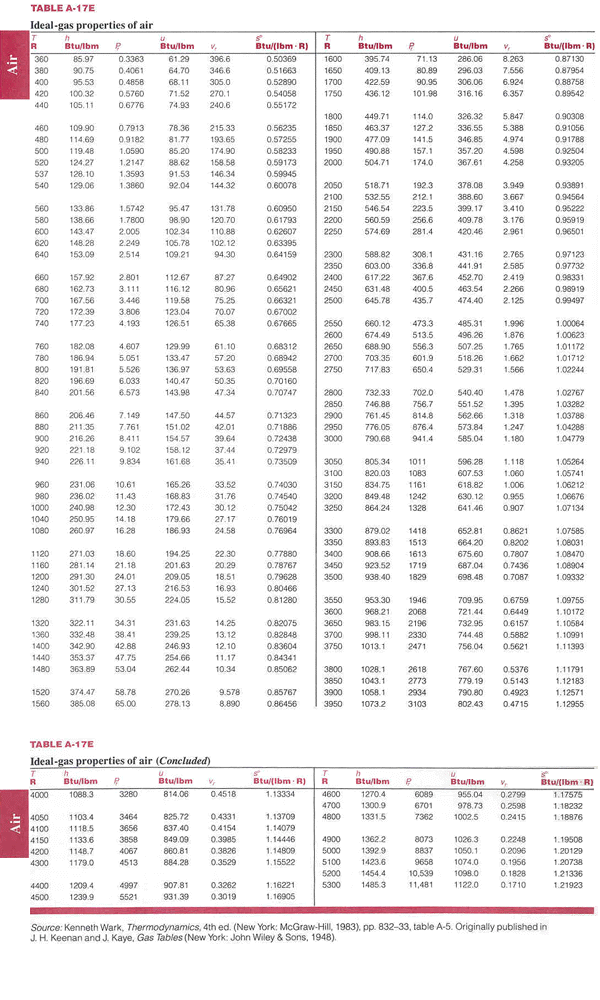
Untitled Document
L14 Physics Of Dry Air And Moist Air Ppt Download

Ideal Gas Law Wikipedia

Ideal Gas Law For Moist Air Ppt Download
Untitled Document
Modelica Media Air Dryairnasa

Property Table Air Ideal Gas Properties Pdf 924 Property Tables And Charts Table A 17 Ideal Gas Properties Of Air T H U S T H U 5 K Kj Kg P Kj Kg V Course Hero

Hot Air Balloon Ideal Gas Law Problem Youtube

Dv Mrth Ln 2 Solving And Inserting Andre Taulani Academia Edu
Ideal Gas Law
:max_bytes(150000):strip_icc()/colorful-hot-air-balloons-599718950-587670d83df78c17b648074b.jpg)
Gay Lussac S Ideal Gas Law Examples

Solved 1 Problem 1 Air Ideal Gas At Pressure 100 Kpa A Chegg Com

Saved By An Air Bag Real World Chemistry Ck 12 Foundation

Chemistry Lab Making Of An Air Bag Ideal Gas Law Stoichiometry Tpt

Ideal Gas Law Calculator Pressure Volume Temperature Amount Thermodynamics Heat Online Unit Converters

Ideal Gas Law Wikipedia
Air Density The Pillar Of Performance Revealed With Gale Banks

Balloon Power Energy Ideal Gas Law Power Energy Thermodynamics
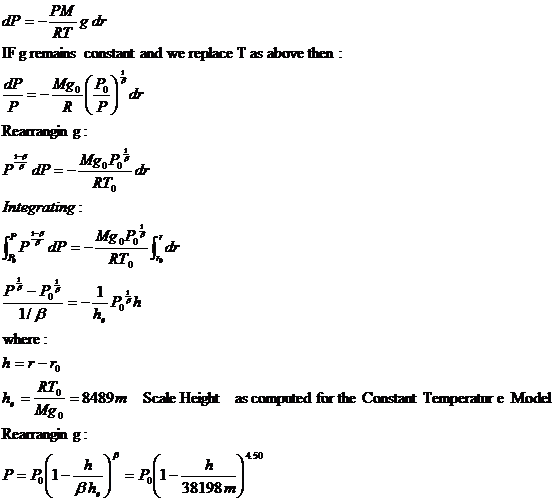
Meteorological Model
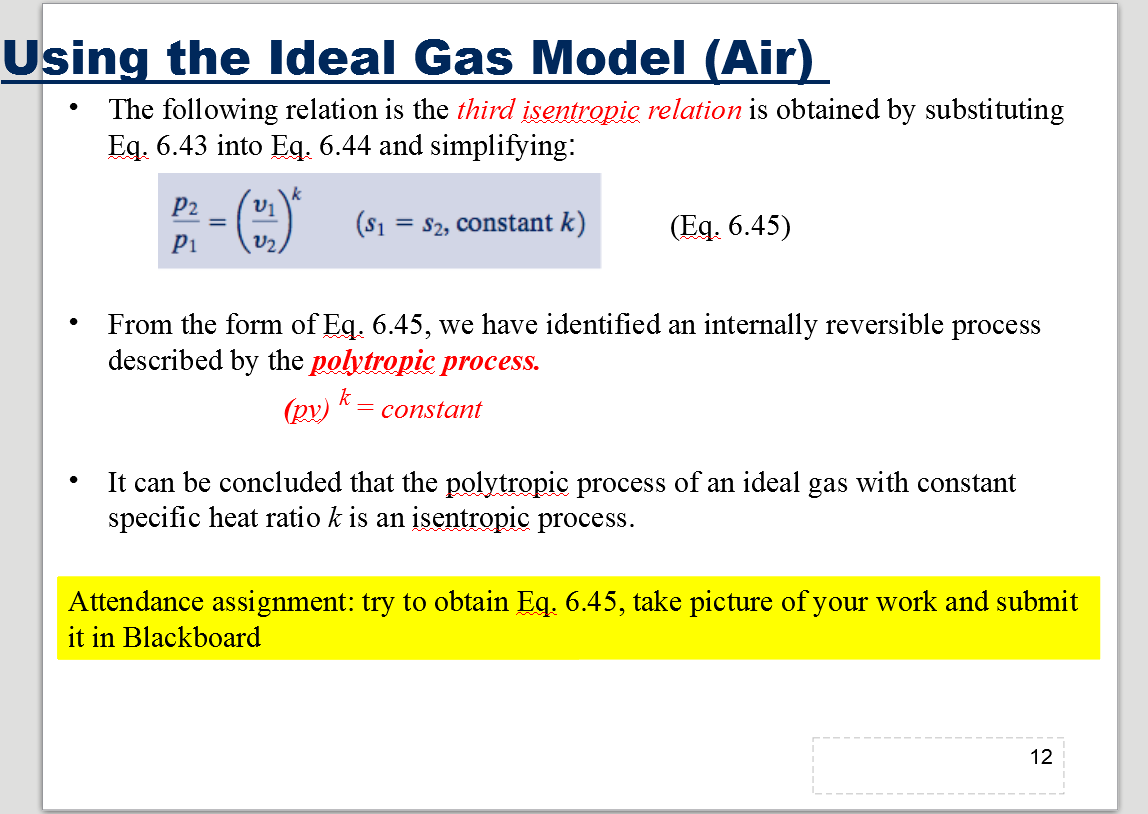
Solved Using The Ideal Gas Model Air The Following Re Chegg Com
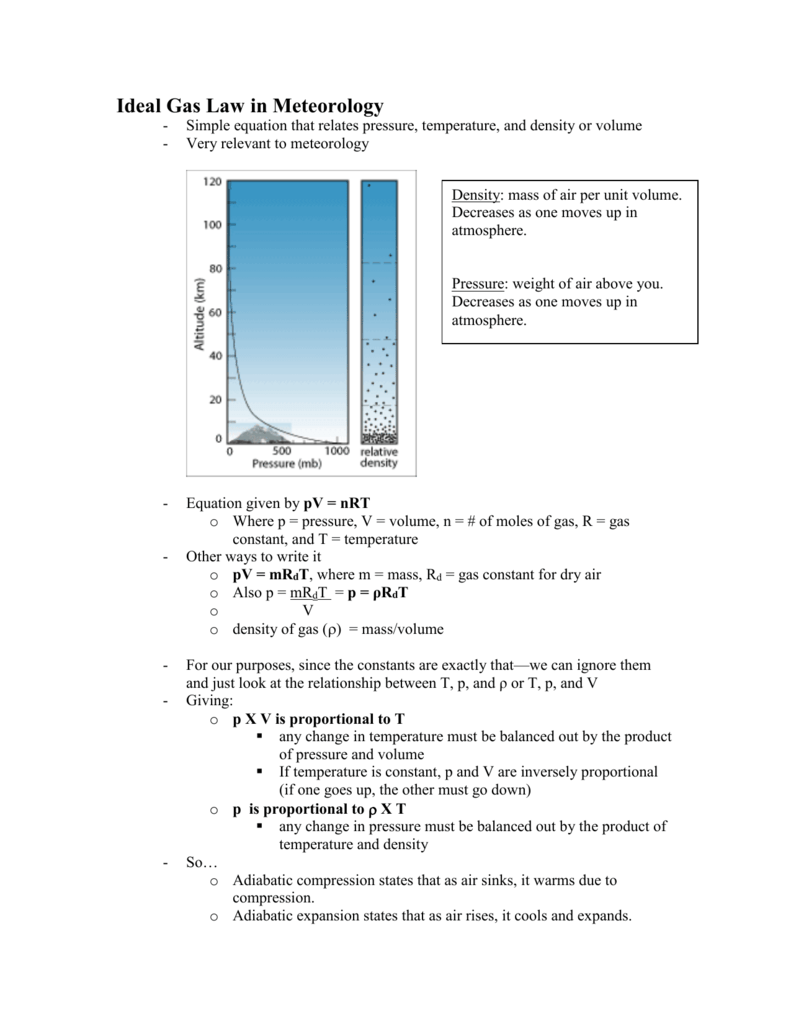
Ideal Gas Law In Meteorology
Solved Create Program Uses Linear Interpolation Find Interpolated Properties Air Ideal Gas Need L Q

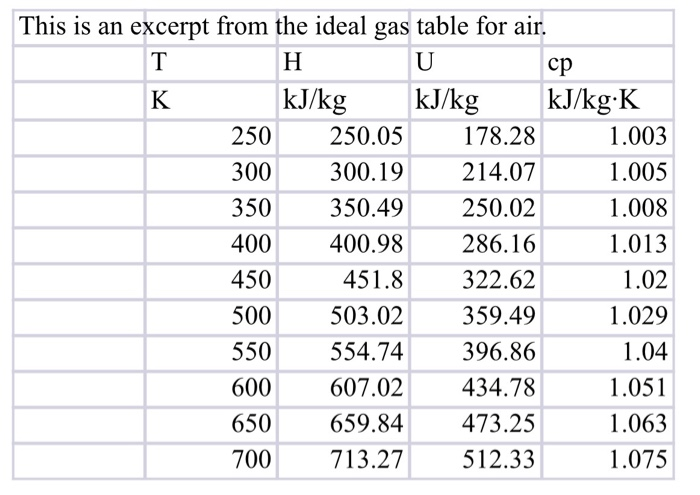
Ideal Gas Table For Air
Lecture 6 Ideal Gas Law Rising And Sinking Air
Is Air Considered An Ideal Gas Why Or Why Not Quora

13 3 The Ideal Gas Law Texas Gateway
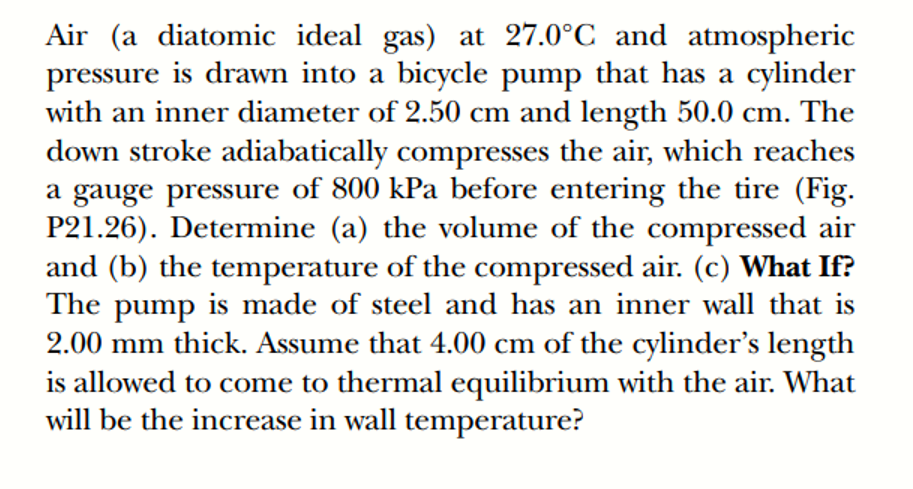
Answered Air A Diatomic Ideal Gas At 27 0 C Bartleby
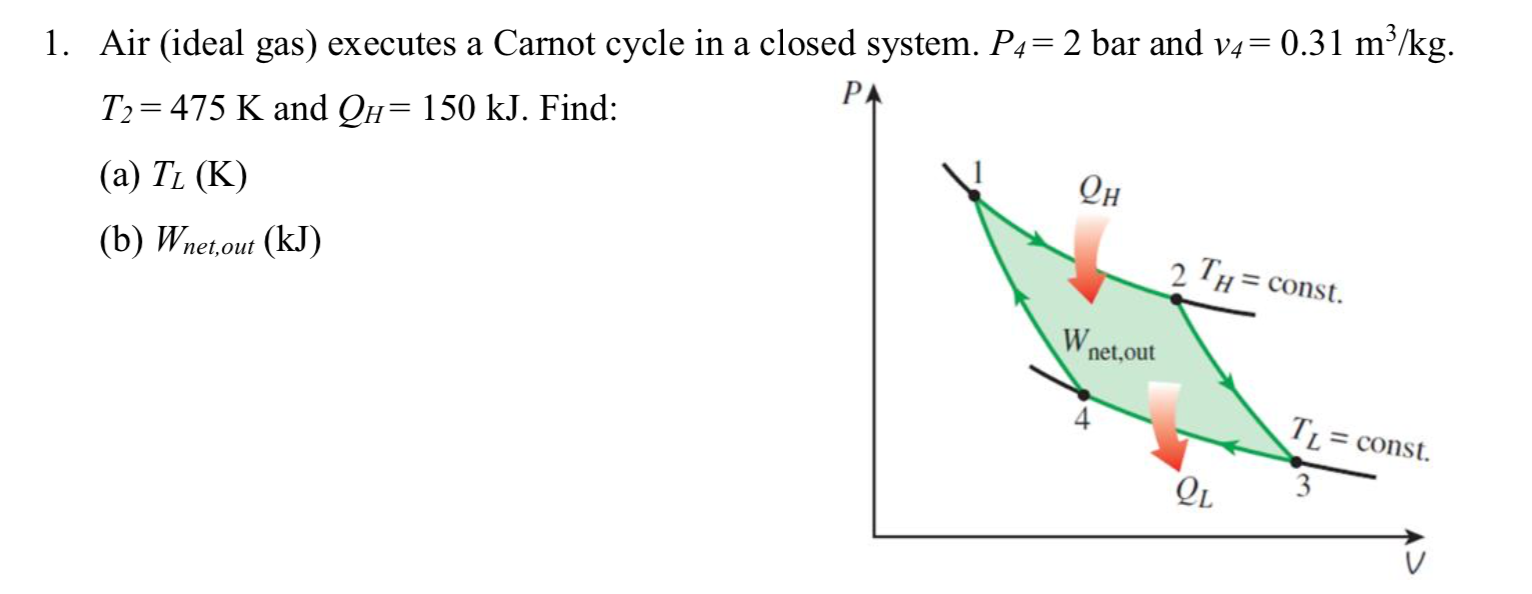
Solved 1 Air Ideal Gas Executes A Carnot Cycle In A Cl Chegg Com

Thermodynamics 4 4 Ideal Gas Specific Heat Example 1 Youtube

Solved Problem 6 Ideal Gas Law Application And Energy B Chegg Com

Ideal Gas Law A History Of The Pneumatic Sciences The Institution For Science Advancement

1 The Ideal Gas Eos Can Be Written In Many Different Ways Ppt Download

How To Calculate Air Density

Gas Behaviour And Properties Britannica

Gas Behaviour And Properties Britannica

Why Sometimes We Can Find P Rgrt Instead Of P Rrt For Ideal Gas Physics Stack Exchange

Ch7 Lesson E Page 3 Pr And Vr Ideal Gas Property Tables

Solved 7 16 Employing The Ideal Gas Model Determine The Change In Transtutors

Ideal Gas Law Ck 12 Foundation

Answered Air Treated As An Ideal Gas And Having Bartleby

Why Can We Treat Air At Ambient Condition As Ideal Gas Youtube

Solved The Entropy Component Presented On The Air Ideal G Chegg Com

Using The Ideal Gas Relationships
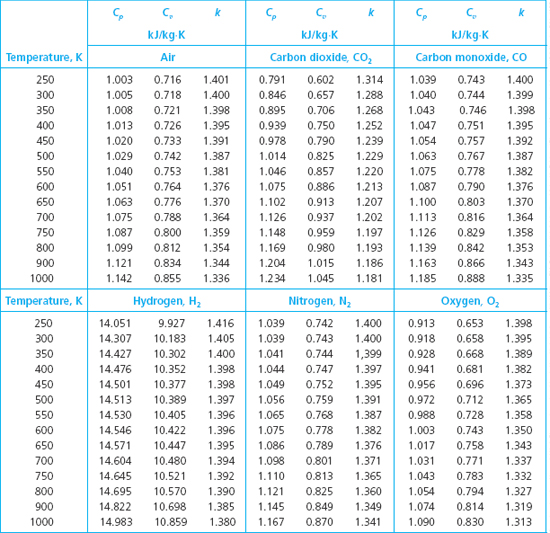
Solved Rework The Following Problems Assuming Variable Specific H Chegg Com

One Kilogram Of Air As An Ideal Gas Executes A Carnot Power Cycle Having A Thermal Efficiency Of 50 The Heat Transfer To The Air During The Isothermal Expansion Is 50 Kj

9 1 An Ideal Gas Flows Adiabatically Through A Duct At Section 1 P1
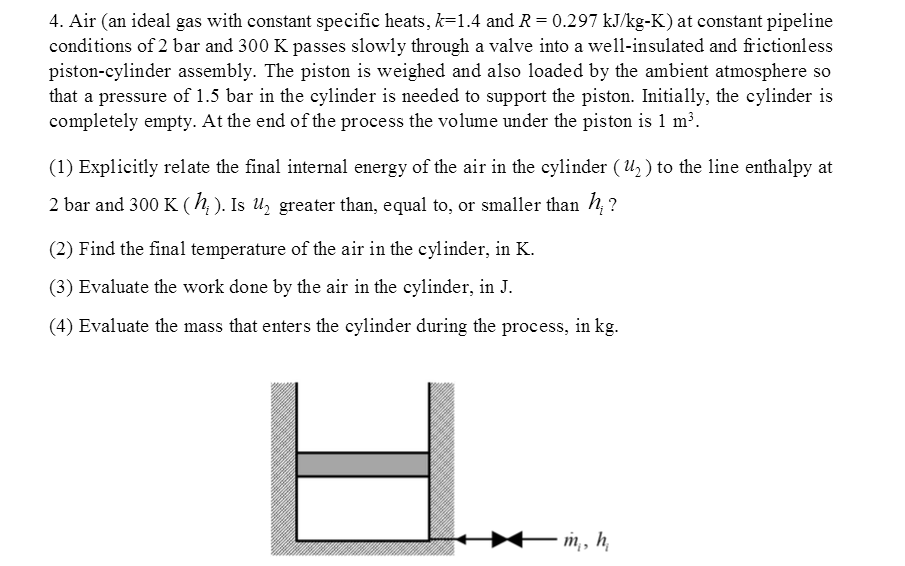
Solved Air An Ideal Gas With Constant Specific Heats K Chegg Com
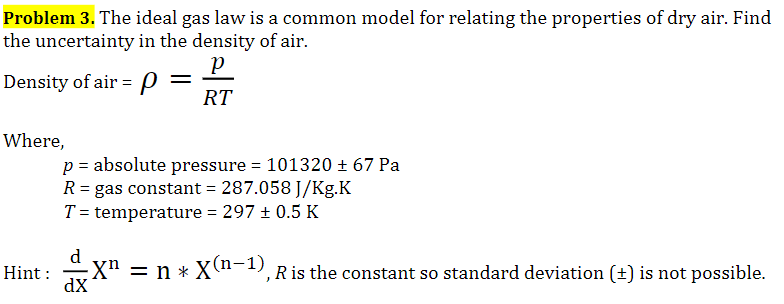
Solved Problem 3 The Ideal Gas Law Is A Common Model For Chegg Com
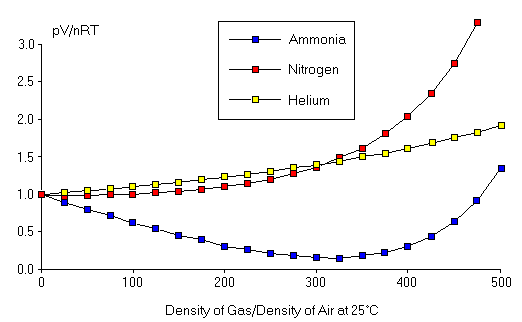
Validity Of The Ideal Gas Law

Ideal Gases
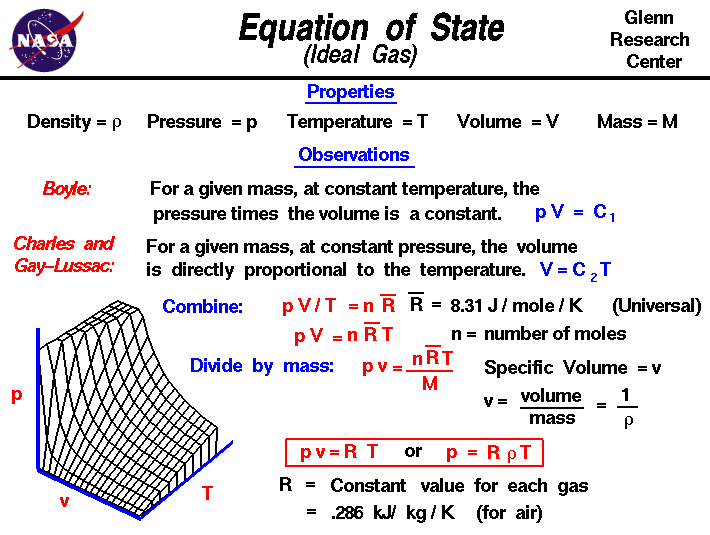
Equation Of State
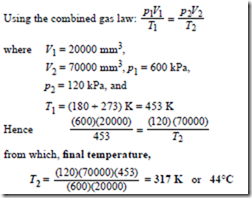
Ideal Gas Laws Worked Problems On The Characteristic Gas Equation And Further Worked Problems On The Characteristic Gas Equation Hvac Machinery
Is Air Considered An Ideal Gas Why Or Why Not Quora
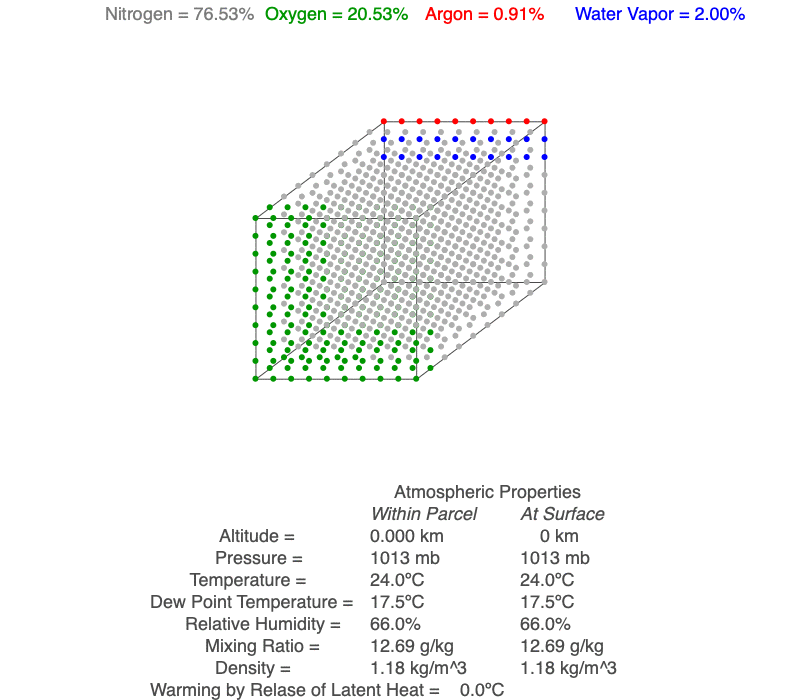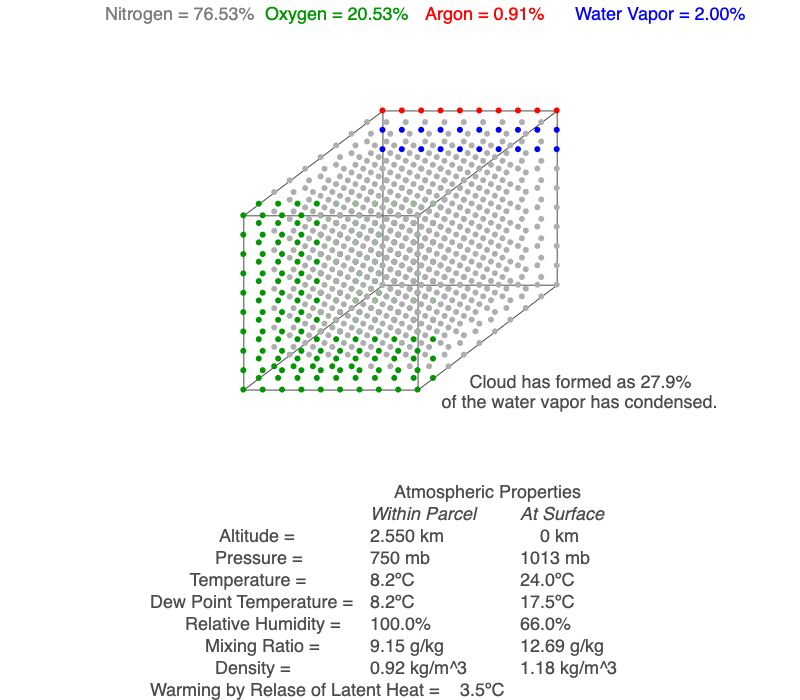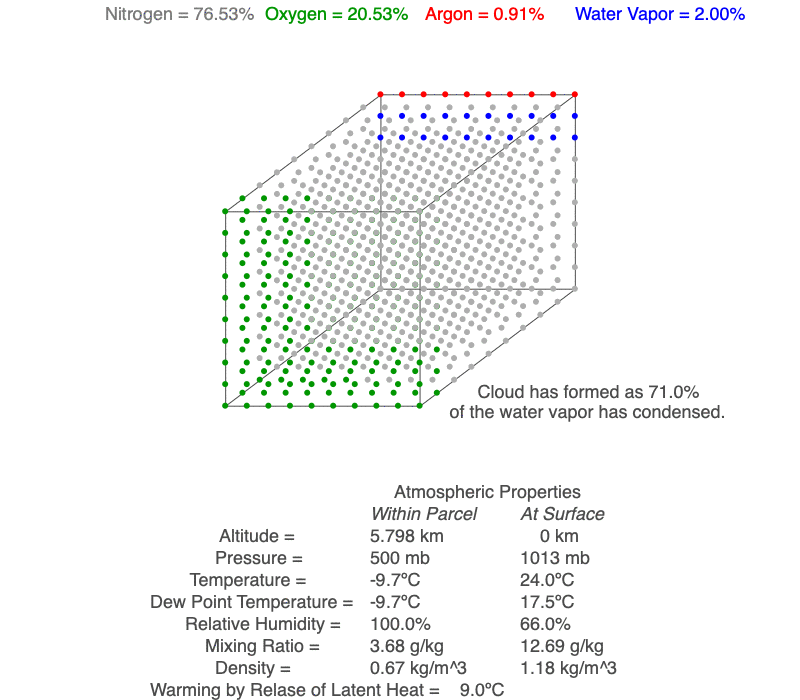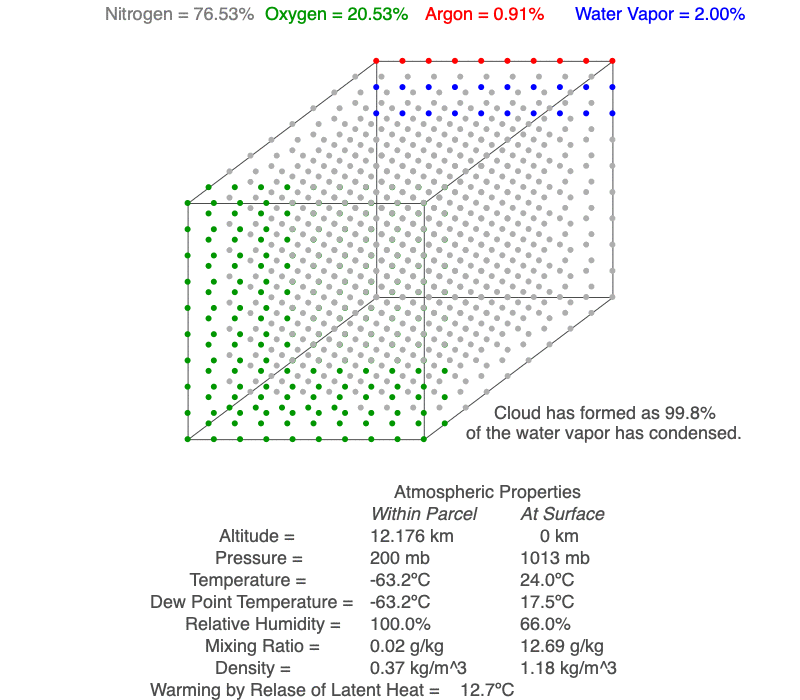
Ideal Gas Law And Clouds Science Pickle

Solved Air As An Ideal Gas Is Compressed From A State Whe Chegg Com

Table A 17 Ideal Gas Properties Of Air 936 I Tharmodynamics Table A L L Ideal Gas Properties Of Air O T H U 5 T H U S K Kj Kg P Lekg V Lekg K K
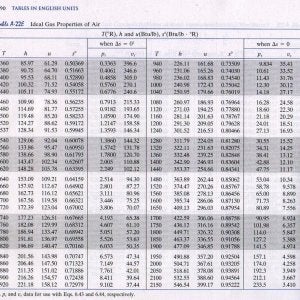
Table A 22e Ideal Gas Properties Of Air The Diesel Stop
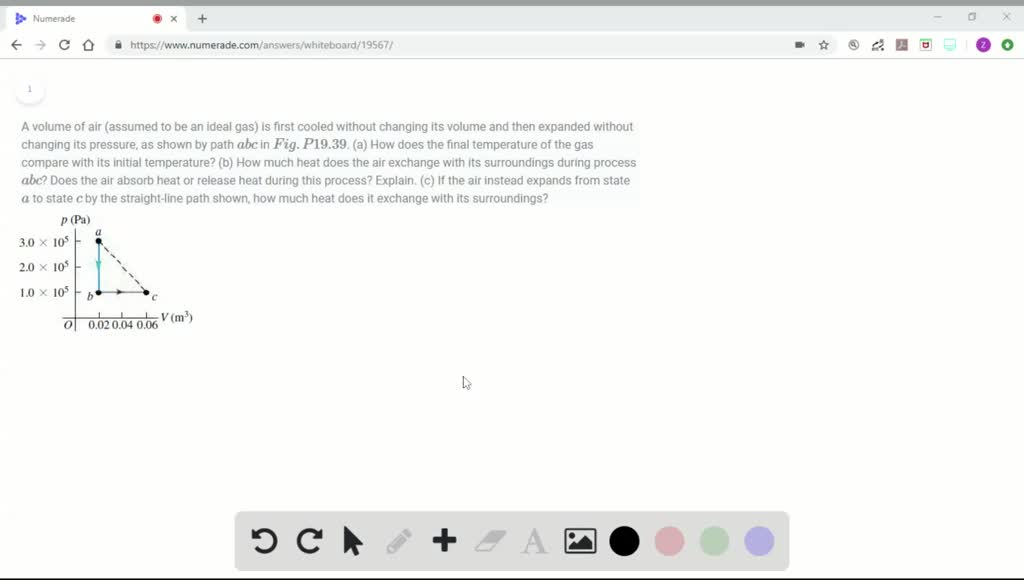
Solved A Volume Of Air Assumed To Be An Ideal Ga

Show That The Internal Energy Of The Air Treated As An Ideal Gas Contained Youtube
Is Air Considered An Ideal Gas Why Or Why Not Quora




